Methods A total of 60 patients (60 eyes) with moderate-to-severe dry eye associated with type 2 diabetes who visited the Ophthalmology Clinic of Peking University Third Hospital between October 2021 and August 2023 were selected. They were divided into a control group and an experimental group using a random number table, with 30 patients (30 eyes) in each group. Among them, there were 19 males (19 eyes) and 41 females (41 eyes) with a mean age of (65.5±8.7) years (ranging from 53 to 79 years). The control group received 0.1% sodium hyaluronate eye drops three times daily, while the experimental group received 0.1% sodium hyaluronate eye drops three times daily combined with 0.05% CsA eye drops twice daily. Before treatment, as well as at 1 month and 3 months after treatment, patients underwent slit-lamp examination, tear film breakup time (BUT) measurement, corneal fluorescein staining (CFS) scoring, lissamine green (LG) staining scoring, corneal sensitivity testing, Schirmer′s test Ⅰ (SⅠt), ocular surface disease index (OSDI) questionnaire assessment, and tear inflammatory factor testing. Age, OSDI scores, and SⅠt were expressed as 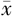 ±s. Comparisons before and after treatment were performed using paired t-test, for intergroup were conducted using independent samples t-test, and within-group and between-group comparisons were analyzed using two-way ANOVA. BUT, CFS scores, mean corneal sensitivity, and LG scores were expressed as median M(Q1, Q3). Comparisons before and after treatment and between groups were performed using rank-sum tests, while within-group and between-group differences were assessed using the Scheirer-Ray-Hare test.
±s. Comparisons before and after treatment were performed using paired t-test, for intergroup were conducted using independent samples t-test, and within-group and between-group comparisons were analyzed using two-way ANOVA. BUT, CFS scores, mean corneal sensitivity, and LG scores were expressed as median M(Q1, Q3). Comparisons before and after treatment and between groups were performed using rank-sum tests, while within-group and between-group differences were assessed using the Scheirer-Ray-Hare test.
Result In the experimental group, OSDI scores, BUT, CFS scores, SⅠt, mean corneal sensitivity, and LG scores before treatment, after treatment for 1 month, 3 months were as follows: (35.05±12.45)points, 4.00(2.00, 6.50) s, 0.50(0.00, 3.75)points, (13.70±8.56) mm, 5.45(5.20, 5.80)points, 0.00(0.00, 2.00)points, (26.06±10.87)points, 4.00(3.00, 5.50) s, 1.00(0.00, 3.25)points, (14.03±9.00) mm, 6.00(5.60, 6.00)points, 0.00(0.00, 2.00)points, (10.98±10.61)points, 6.50(4.00, 9.00)s, 0.0(0.00, 2.25)points, (13.61±7.32) mm, 6.00(5.90, 6.00)points, 0.00(0.00, 1.00)points, respectively. In the control group, the corresponding values were (31.43±11.11)points, 3.00(2.75, 6.00)s, 1.00(0.00, 3.00)points, (12.30±7.87) mm, 5.75(5.38, 6.00)points, 0.00(0.00, 2.00)points, (31.33±14.64)points, 3.00(2.00, 7.00)s, 1.00(0.00, 3.00)points, (12.23±8.40) mm, 5.75(5.20, 6.00)points, 0.00(0.00, 1.00)points, and (12.97±13.69)points, 4.00(3.00, 6.25) s, 0.00(0.00, 3.00)points, (11.40±7.97) mm, 6.00(5.80, 6.00)points, 0.00(0.00, 2.00)points, respectively. Two-way ANOVA revealed statistically significant differences in OSDI scores within groups across timepoints (F=63.45, P<0.05), but no significant differences between groups or in interaction effects (F=0.20, 2.59; P>0.05). There were significant differences within groups and in interaction effects in BUT (H= 6.92, 4.09; P<0.05), but not between groups (H= 1.36, P>0.05). No significant differences were found in CFS scores, SⅠt, mean corneal sensitivity, or LG scores between groups, within groups, or in interaction effects (H= 0.04, 1.96, 2.60, 2.05, 0.10, 0.22, 1.85, 1.19, 1.70, 0.16, 0.52, 2.12; P>0.05). After treatment for 1 month, there was no significant difference in BUT between the two groups (Z=0.68, P>0.05). However, after treatment for 3 months, the difference in BUT between the two groups was statistically significant (Z=2.19, P<0.05).